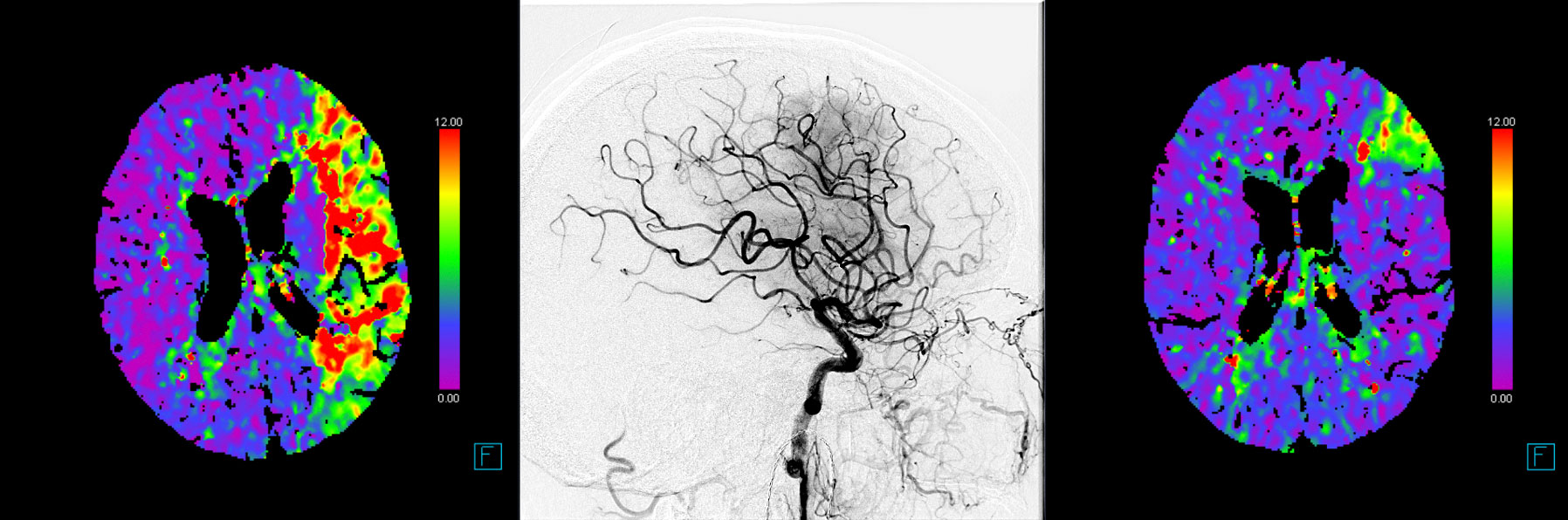
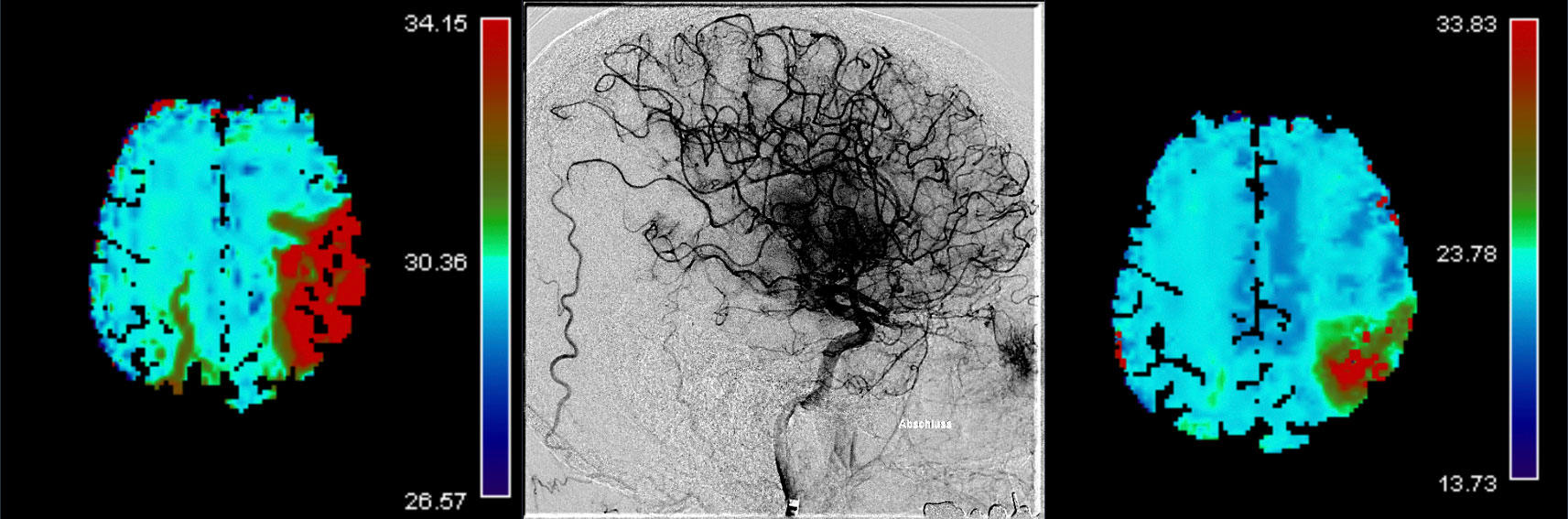
Current state of evidence
After publication of seven randomized-controlled trials, mechanical thrombectomy (MT) is the current standard of care in patients presenting with an acute ischemic stroke caused by a large vessel occlusion in the anterior circulation. Despite technical advances and overall increasing reperfusion quality, still 40% of patients show a non-complete reperfusion after MT (Figure 1 and 2).1–7
Pooled patient-level data of the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) collaboration showed strong association between achieved reperfusion grade and functional outcome.8 This difference was also observed between successful reperfusion grades of TICI 2b, 2c and 3.9,10These findings have also been corroborated by two meta-analyses of observational data which included over 2000 patients.11,12
Acknowledging these outcome differences across successful reperfusion grades, European Stroke Organisation (ESO) and European Society of Minimally Invasive Neurological Therapy (ESMINT) current guidelines state that “interventionalists should attempt a TICI3 reperfusion, if achievable with reasonable safety”.13 However, distal vessel occlusions causing non-complete reperfusions are often not manageable with additional mechanical rescue maneuvers, but may be recanalized with fibrinolytic agents.14–17 Fibrinolytics have shown to be particular effective in dissolving small clots,18 and recent analyses revealed that the administration of intra-arterial fibrinolytic may improve reperfusion grades after non-complete MT with increased clinical benefit.14,15
TNK Background
Tenecteplase (TNK) is a fibrin-specific plasminogen activator thrombolytic agent showing promising results in acute ischemic stroke treatment. TNK has advantageous pharmacological characteristics and ease of administration when compared to tPA i.e. bolus administration alone, instead of bolus followed by a continuous infusion.19 Specifically, TNK has higher fibrin specificity, increased resistance to plasminogen activator, and longer half-life. Therefore, TNK harbors the potential to increase the feasibility of IA administration and potentially reduce endovascular complication (Figure 3). Several randomized-controlled clinical trials have also evaluated the use of intravenous TNK in patients presenting with an acute ischemic stroke.20,21
According to the recent 2019 ESO/ESMINT guidelines on MT, seven out of eleven experts recommended TNK over alteplase if the vessel occlusion status is known at the time point of decision-making.22 As of now, no prospective evaluation of administering intra-arterial TNK in patients with incomplete reperfusion is available.
Trial design
TECNO is a multicenter, prospective, randomized, open label, blinded endpoint (PROBE) trial utilizing an adaptive statistical design comparing the rates of early and late reperfusion in patients with incomplete mechanical thrombectomy treated either by IA TNK or by best medical treatment BMT. This trial has two balanced treatment arms, with the IA TNK being the experimental arm. The standard arm is defined as BMT after incomplete MT. 156 patients will be randomly assigned to either the standard or the experimental arm with a ratio of 1:1 (78 patients per group).
Randomization
All patients older than 18 years with an acute ischemic stroke and an imaging confirmed occlusion of a proximal anterior circulation who undergo MT after which they have an incomplete reperfusion will be considered for randomization. Patients randomized to intervention will receive IA TNK using a standard approved microcatheter. Patients randomized to control will receive best medical treatment i.e. standard of care treatment as per current ESO guidelines (Figure 4). The inclusion of patients will start in July 2022 and will stop after randomizing 156 patients, which is expected to happen in October 2024.
Outcomes
The two primary efficacy outcomes are early reperfusion (25 minutes after randomization on angiography) and late reperfusion (24h±6h after randomization on MR/CT perfusion imaging), defined as the absence of a perfusion deficit which was observed on the final angiography imaging before randomization. Co-primary outcome has been chosen because there are large uncertainties regarding the timing of intra-arterial thrombolysis effect in distal occlusions after MT.
Secondary outcomes will be evaluating stroke severity, degree of disability, life quality and mortality at different time points after the ischemic event.
Key inclusion and exclusion criteria
Key inclusion criteria
- Informed consent
- Age ≥18 years
- Clinical signs consistent with an acute ischemic stroke
- Patient had an initial large vessel occlusion in the anterior circulation defined as intracranial ICA, M1 or M2.
- Patient has undergone endovascular stroke treatment
- Onset to randomization no later than < 345 minutes after symptom-onset/last-seen well.
- Incomplete reperfusion defined as
- For ICA/M1: TICI2b (50-89%) reperfusion after endovascular treatment without mechanically amendable target-occlusion (as per definition by the interventionalist).
- For M2: TICI2a or TICI2b (1-89%) reperfusion after endovascular treatment without mechanically amendable target-occlusion (as per definition by the interventionalist).
- ICA/M1/M2 with TICI3 reperfusion (MCA territory) but emboli to the ACA territory without mechanically amendable target-occlusion (as per definition by the interventionalist).
- Signs of early ischemic changes of non-contrast CT Alberta Stroke Program Early CT Score (ASPECTS) ≥5 (for DWI-ASPECTS ≥ 4, for DWI-ASECTS: a region must have diffusion abnormality in 20% or more of its volume to be considered DWI-ASPECTS positive)
Key exclusion criteria
- Acute intracranial hemorrhage
- Contraindication to MRI (e.g. pacemaker)
- Patients with both, anterior and middle cerebral artery embolizations during the procedure
- Tandem occlusion requiring cervical stenting
- Any severe bleeding within the past 6 months
- Major surgery in the past 2 months
- Intake of direct oral anticoagulants <12h or Vitamin K Antagonist with INR >1.3
- Platelets < 50,000
- Non-controlled hypertension (defined as SBP >185 mmHg or DBP >110 mmHg refractory to treatment)
Trial structure
Steering Committee
PD Dr. Johannes Kaesmacher
Prof. Dr. Urs Fischer
Prof. Dr. Jan Gralla
Prof. Dr. Mira Katan
Prof. Dr. Pasquale Mordasini
Prof. Dr. Zsolt Kulcsar
Prof. Dr. Marios Psychogios
Prof. Dr. Mikael Mazighi
Prof. Dr. Daniel Strbian
Prof. Dr. Götz Thomalla
Prof. Dr. Robin Lemmens
Data Safety Monitoring Board
Prof. Dr. Bruce Campbell
Prof. Tim Friede
Prof. Dr. Wim van Zwam
Literature
- Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJH, et al. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. N. Engl. J. Med. 2015;372:11–20.
- Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, et al. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. N. Engl. J. Med. 2015;372:2285–2295.
- Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. N. Engl. J. Med. 2015;372:1009–1018.
- Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N. Engl. J. Med. 2015;372:1019–1030.
- Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. N. Engl. J. Med. 2015;372:2296–2306.
- Fischer U, Kaesmacher J, S Plattner P, Bütikofer L, Mordasini P, Deppeler S, Cognard C, Pereira VM, Siddiqui AH, Froehler MT, et al. SWIFT DIRECT: SolitaireTM With the Intention For Thrombectomy Plus Intravenous t-PA Versus DIRECT SolitaireTM Stent-retriever Thrombectomy in Acute Anterior Circulation Stroke: Methodology of a randomized, controlled, multicentre study. Int. J. Stroke. 2021;174749302110487.
- Mitchell PJ, Yan B. DIRECT-SAFE: A randomized controlled trial of DIRECT endovascular clot retrieval versus standard bridging thrombolysis with endovascular clot retrieval. ClinicalTrials.gov [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT03494920
- Goyal M, Menon BK, Van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CBLM, Van Der Lugt A, De Miquel MA, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731.
- Goyal M, Fargen KM, Turk AS, Mocco J, Liebeskind DS, Frei D, Demchuk AM. 2C or not 2C: Defining an improved revascularization grading scale and the need for standardization of angiography outcomes in stroke trials. J. Neurointerv. Surg. 2014;6:83–86.
- Liebeskind DS, Bracard S, Guillemin F, Jahan R, Jovin TG, Majoie CBLM, Mitchell PJ, Van Der Lugt A, Menon BK, San Román L, et al. ETICI reperfusion: Defining success in endovascular stroke therapy. J. Neurointerv. Surg. 2019;11:433–438.
- Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, Poppe AY, Buck BH, Field TS, Dowlatshahi D, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395:878–887.
- Kaesmacher J, Dobrocky T, Heldner MR, Bellwald S, Mosimann PJ, Mordasini P, Bigi S, Arnold M, Gralla J, Fischer U. Systematic review and meta-analysis on outcome differences among patients with TICI2b versus TICI3 reperfusions: Success revisited. J. Neurol. Neurosurg. Psychiatry. 2018;89:910–917.
- Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, De Vries J, White P, et al. European Stroke Organisation (ESO) – European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischemic Stroke. J. Neurointerv. Surg. 2019;1–30.
- Kaesmacher J, Bellwald S, Dobrocky T, Meinel TR, Piechowiak EI, Goeldlin M, Kurmann CC, Heldner MR, Jung S, Mordasini P, et al. Safety and Efficacy of Intra-arterial Urokinase after Failed, Unsuccessful, or Incomplete Mechanical Thrombectomy in Anterior Circulation Large-Vessel Occlusion Stroke. JAMA Neurol. 2020;77:318–326.
- Zaidi SF, Castonguay AC, Jumaa MA, Malisch TW, Linfante I, Marden FA, Abraham MG, Chebl AB, Novakovic R, Taqi MA, et al. Intraarterial Thrombolysis as Rescue Therapy for Large Vessel Occlusions. Stroke. 2019;50:1003–1006.
- Anadani M, Ajinkya S, Alawieh A, Vargas J, Chatterjee A, Turk A, Spiotta AM. Intra-Arterial Tissue Plasminogen Activator Is a Safe Rescue Therapy with Mechanical Thrombectomy. World Neurosurg. 2018;123:e604–e608.
- Lee M, Hong K, Saver JL, Fibrinolysis IA. Efficacy of Intra-Arterial Fibrinolysis for Acute Ischemic Stroke Meta-Analysis of Randomized Controlled Trials. 2010;932–937.
- Rahme R, Abruzzo TA, Martin RH, Tomsick TA, Ringer AJ, Furlan AJ, Carrozzella JA, Khatri P. Is intra-arterial thrombolysis beneficial for M2 occlusions? Subgroup analysis of the PROACT-II trial. Stroke. 2013;44:240–242.
- Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, Yan B, Bush SJ, Dewey HM, Thijs V, et al. Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke. N. Engl. J. Med. 2018;378:1573–1582.
- Warach SJ, Dula AN, Milling TJ. Tenecteplase thrombolysis for acute ischemic stroke. Stroke. 2020;3440–3451.
- Coutts SB, Berge E, Campbell BC, Muir KW, Parsons MW. Tenecteplase for the treatment of acute ischemic stroke: A review of completed and ongoing randomized controlled trials. Int. J. Stroke. 2018;13:885–892.
- Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, de Vries J, White P, et al. European Stroke Organisation (ESO) – European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE). Eur. Stroke J. 2019;4:6–12.