Safety and Efficacy of Intra-Arterial Tenecteplase for Non-Complete Reperfusion of Intracranial Occlusions
Short description
TECNO is a multicenter, prospective, randomized, open label, blinded endpoint (PROBE) trial utilizing an adaptive statistical design comparing the rates of early and late reperfusion in patients with incomplete mechanical thrombectomy treated either by intraarterial (IA) tenectaplase (TNK) or by best medical treatment (BMT). This trial has two balanced treatment arms, with the IA TNK being the experimental arm. The standard arm is defined as BMT after incomplete MT. 156 patients will be randomly assigned to either the standard or the experimental arm with a ratio of 1:1 (78 patients per group).
Objective
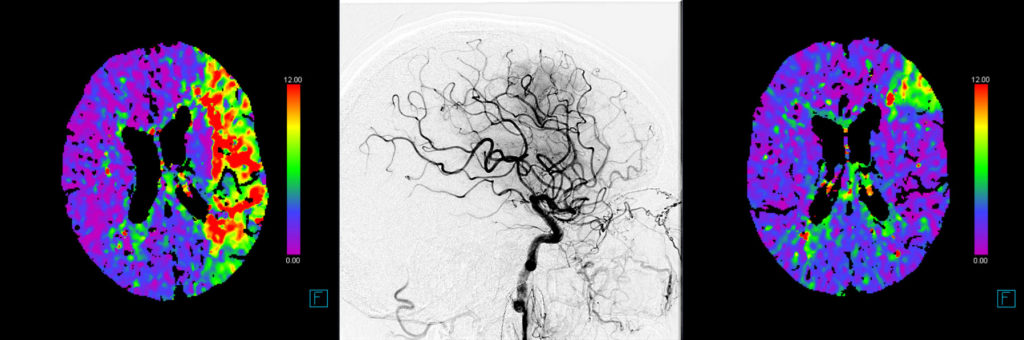
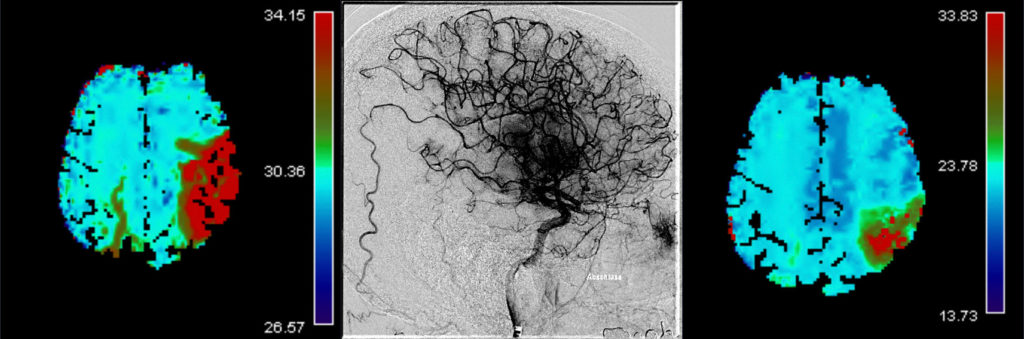
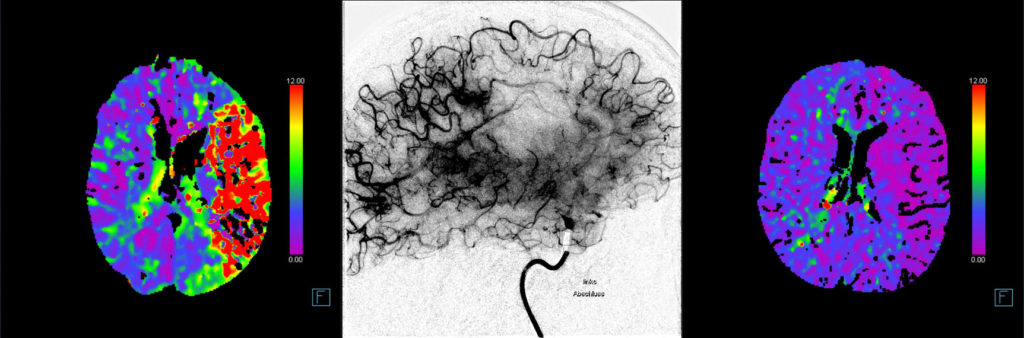
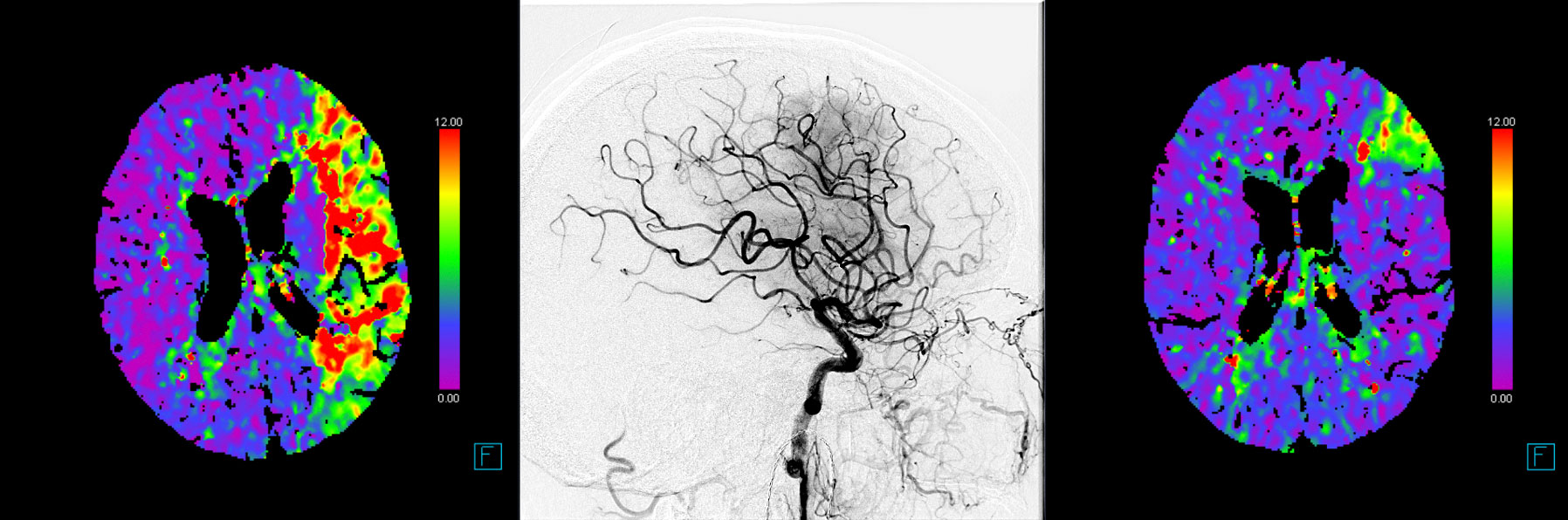
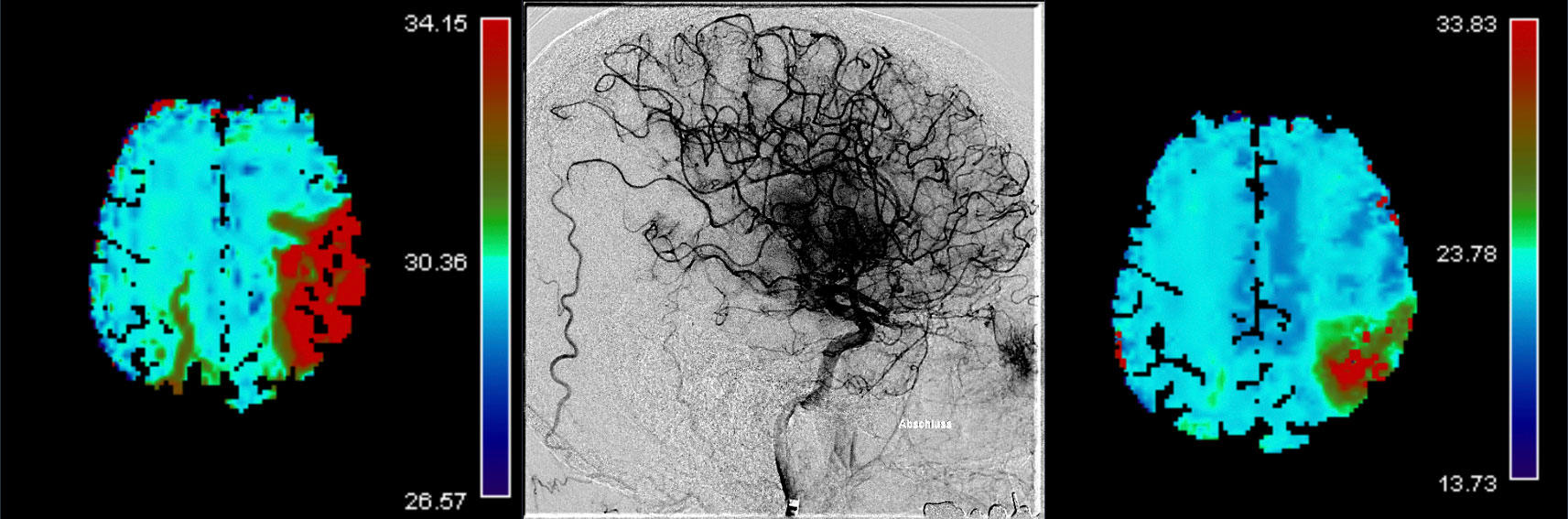
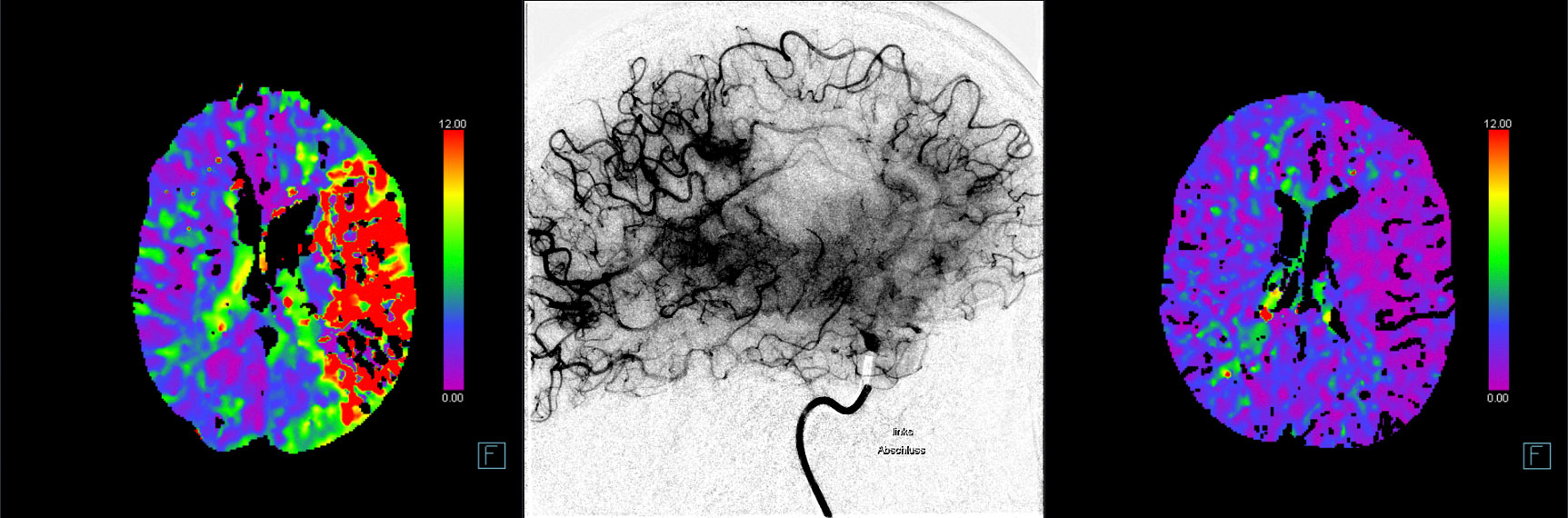
The primary efficacy outcomes are early and late reperfusion, as defined by reperfusion improvement on angiography images 25 minutes after randomization, or complete reperfusion on 24h±6h MR/CT perfusion imaging, respectively.
Secondary objectives are superior clinical and technical efficacy in outcome of patients treated with additional intra-arterial tenecteplase, when compared to patients undergoing best medical treatment after incomplete mechanical thrombectomy.
Principal investigators
PD Dr.med. Johannes Kaesmacher
Department of Neuroradiology
Prof. Dr. med. Urs Fischer
Department of Neurology
University Hospital Bern Inselspital
Bern, Switzerland